
In other words, if we supply 10 MJ ( Q 1) of heat to produce work ( W), we can only develop less than 10 MJ of work, because the heat rejected to the sink, Q 2, cannot be zero. One definition of the second law limits the amount of work that can be produced. It should be noted that when thermodynamic systems such as a heat engine operate in a cycle, this results in the initial and final states being identical. A heat engine is a device operating in a cycle, producing work from a heat source and rejecting heat to a heat sink as shown in Fig.
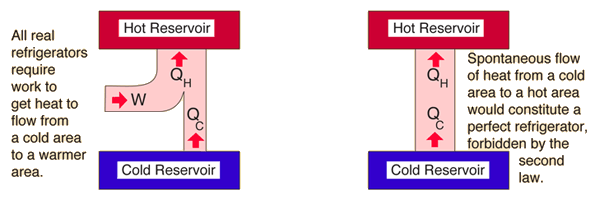
The second law of thermodynamics is normally associated with a heat engine. Therefore, another formulation, or a usable quantity, has to be derived, which will be demonstrated in the following section. Stated in this form, the second law cannot be applied conveniently to practical problems. It is not possible that, as the only result of a process, an amount of heat that flows from the surroundings to the system is converted into work, which is performed by the system. These kinds of observations have resulted in the second law of thermodynamics which may be stated as follows: a flow of heat from the surroundings to the system and performance of work by the system on the paddle wheel, however, has never been observed, although this process is not in conflict with the first law. This implies that the work performed on the system is converted into heat that is supplied by the system to the surroundings. The observation is in accordance with the first law: if the state of the system does not change (the temperature is constant), the internal energy of the system is constant and, from equation (8), it is found that the amount of heat which flows from the system to the surroundings is equal to the amount of work performed on the system. This can only be accomplished by a flow of heat from the system to the surroundings. Suppose work is performed on the paddle wheel, but the system is kept at constant temperature. In section 3.3, it was demonstrated that a system, consisting of an amount of water in which a paddle wheel is placed, may be heated by performing work on the paddle wheel (i.e. The experience that leads to the second law is the following. For didactic purposes, however, the first law is usually treated first. The second law of thermodynamics was developed during the investigations of Sadi Carnot (1796 - 1832) and dates from before the first law. van Ekeren, in Handbook of Thermal Analysis and Calorimetry, 1998 4.1 Negative formulation of the second law


 0 kommentar(er)
0 kommentar(er)
